We automate and validate your process
Our automation roadmap
To achieve a product fit for the market and contain costs, it is crucial to investigate and compare, at a very early stage, existing options for automation, whether already available or to be developed ad-hoc. Automation is a key element, as it increases bioprocess efficiency and reproducibility, and allows scale-up/out. In addition, it reduces personnel intervention and the need for grade A space (in case of closed systems). Our team of experts will guide you through analysis and feasibility study phases that will result in an automated solution tailored on your bioprocess and GMP requirements.
At this stage we analyze your bioprocess and market specificities, develop custom strategies of how to automate it, compare them to each other, and estimate development as well as production costs. Whenever possible, the suggested approaches are based on existing off-the-shelf devices.
The planned experiments are carried out in our lab with your cells. As an example, if the chosen automation approach involves the use tubing for cell transportation, it is verified that cells can be pumped through tubing at different speeds without any consequence.
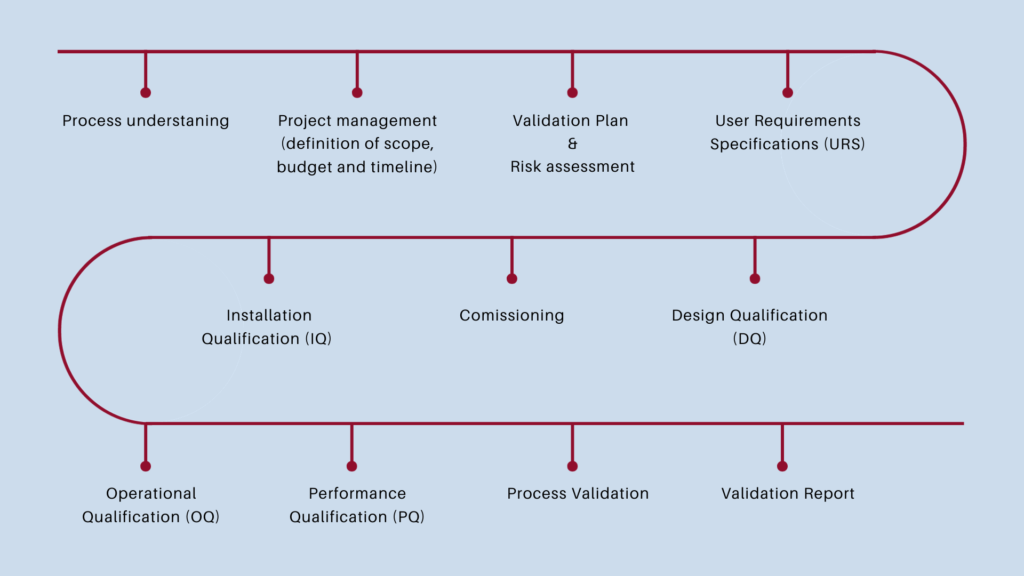
Validation and qualification roadmap
Our recipe to successful GMP compliant process validation & equipment qualification
Your advantages in consulting with us
Experts from different fields

Our team brings at your service experience in many different biotechnology fields – e.g. cell-therapy, tissue engineering, cultivated meat – as well as in hardware, mechanics, and software development.
8+ year of experience
We analyze existing bioprocesses and develop approaches to automated them since 2014. In this time, we have successfully concluded several projects for both industry and academia.
Building the lab of the future

All developed setups and prototypes for feasibility studies are from the start monitored and controlled through the OSPIN App, propelling you in the lab of the future.